RESEARCH
Subunit exchange of hemoglobin Ώ2ΐ2 tetramer
- Subunit exchange between PEGylated and native Hbs
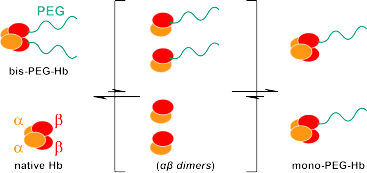
Fig. 1 Dimeric Ώΐ subunit exchange between bis-PEGylated Hb (bis-PEG-Hb) and native Hb
through association-dissociation equilibrium of Ώ2ΐ2 tetramers.
Biomacromolecules 19(8), 3412-3420 (2018)
HDL:10564/3868 in institutional repository (the version post-peer review)
(Abstract)
Various chemical modifications of hemoglobin (Hb) including PEGylation have been investigated to produce red blood cell substitutes. Some of those modifications are designed on the premise that the Ώ2ΐ2 tetrameric structure of Hb is fundamentally stable and that it rarely dissociates into two Ώΐ dimers in a physiological condition. However, in the present work using the gclippingh method we detected and quantitatively analyzed the considerable degree of exchange reaction of Ώΐ subunits between ΐ93Cys-bis-PEGylated and native Hbs through dissociation into Ώΐ dimers and restructuring to Ώ2ΐ2 tetramer in a physiological condition. The equilibrium constant (Keq) of subunit exchange reactions increased from 0.82 to 2.86 with increasing molecular weight of PEG from 2 to 40 kDa, indicating that longer PEG chains enhanced such exchange reaction. The results suggest that the exchange might occur for other modified Hbs even at a practically high concentration for use as a red blood cell substitute.
- Ring-opening polymerization utilizing the association-dissociation equilibrium of Hb units
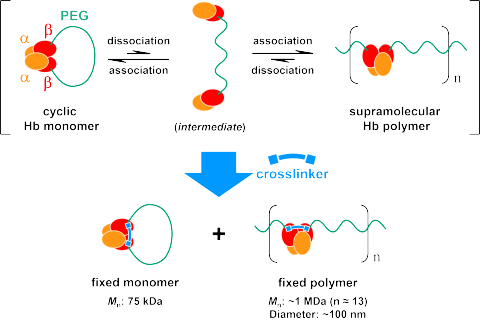
Fig. 2 Ring-opening polymerization of cyclic Hb monomer and subsequernt
crosslinking.
Biomacromolecules 20(4), 1592-1602 (2019) >> Journal Cover Image (Supplementary)
HDL:10564/3869 in institutional repository
(Abstract)
Hemoglobin (Hb), an oxygen-carrying protein, has an Ώ2ΐ2 tetrameric structure that dissociates reversibly into two Ώΐ dimers (Ώ2ΐ2 - 2Ώΐ). We synthesized a cyclic Hb-ring monomer with two ΐ subunits bound through a 10 kDa PEG chain. The monomer induced ring-opening polymerization to produce a supramolecular polymer via inter-subunit interaction of Ώΐ dimers of an Hb molecule at the PEG terminals. Both the ring-closed monomer and the ring-opened supramolecular polymer were then fixed covalently by intramolecular crosslinking of two ΐ subunits. Quantification of fixed products at various monomer concentrations revealed the equilibrium constant (K), a ratio of propagation and depropagation rate constants, as 5.68 mM-1. The average degree of polymerization (DP) increased proportionally, concomitantly with the initial monomer concentration. Hb polymer with DP = 13.2 (Mn = ca. 1 MDa) was obtained by crosslinking at 2.33 mM. Our novel strategy of ring-opening polymerization of Hb will eventually realize a highly aligned and efficiently polymerized Hb for creating artificial oxygen carriers for a clinical use.
Title: Entropy-Driven Supramolecular Ring-Opening Polymerization of a Cyclic Hemoglobin Monomer for Constructing a Hemoglobin-PEG Alternating Polymer with Structural Regularity
Biomacromolecules 32(5), 1944-1954 (2021).
(Abstract)
Our earlier report described that a cyclic hemoglobin (Hb) monomer with two ΐ subunits of a Hb molecule (Ώ2ΐ2) bound through a flexible polyethylene glycol (PEG) chain undergoes reversible supramolecular ring-opening polymerization (S-ROP) to produce a supramolecular Hb polymer with a Hb-PEG alternating structure. In this work, we polymerized cyclic Hb monomers with different ring sizes (2, 5, 10, or 20 kDa PEG) to evaluate the thermodynamics of S-ROP equilibrium. Quantification of the produced supramolecular Hb polymers and the remaining cyclic Hb monomers in the equilibrium state revealed a negligibly small enthalpy change in S-ROP (’Hp < 1 kJ mol-1) and a markedly positive entropy change increasing with the ring size (’Sp = 26.8-33.2 J mol-1 K-1). The results suggest an entropy-driven mechanism in S-ROP: a cyclic Hb monomer with the larger ring size prefers to form a supramolecular Hb polymer. The S-ROP used for this study has the potential to construct submicrometer-sized Hb-PEG alternating polymers having structural regularity.
Banners
SAKAI Laboratory
Postal Code 634-8521
Shijo-cho 840, Kashihara,
Nara, Japan
TEL +81-744-29-8810
FAX +81-744-29-8810